Comments (continue).
VVUS (buy) updated in May, 03 2011 to strong buy
February 05, 2011 by BiotechInvest
Finally all 3 obesity drugs got CRL from FDA. No one got rejection but CRL for OREX asks additional clinical trial i.e. 2-3 years delay. ARNA is still in limbo because of "rat cancer". And VVUS needs to collect the statistical data for Qnexa compound Topiramate:
"The U.S. Food and Drug Administration asked Vivus to examine whether it can use existing databases to determine the risk of oral cleft in children whose mothers took topiramate to prevent migraines, the company said today in a statement. Vivus’s diet pill, Qnexa, combines topiramate with the appetite suppressant phentermine."
Completed studies of Qnexa included 15 births from women who had taken the medicine or topiramate, and no birth defects were reported, Vivus said."
Topiramate Label Warnings
Pregnancy
Only When Necessary: POST-MARKET REPORT OF ORAL CLEFTS/MALE HYPOSPADIAS IN TOPIRAMATE POLYTHERAPY.
Pregnant women who take an epilepsy drug that is also prescribed for migraines may increase the risk of their children having birth defects birth defects, abnormalities in physical or mental structure or function that are present at birth. They range from minor to seriously deforming or life-threatening. A major defect of some type occurs in approximately 3% of all births, doctors warned.
Babies born to women who took topiramate during pregnancy were more likely to have cleft palates (cleft palate, incomplete fusion of bones of the palate). The cleft may be confined to the soft palate at the back of the mouth; it may include the hard palate, or roof of the mouth; or it may extend through the gum and lip, producing a gap in the teeth and a cleft , cleft lips and genital abnormalities, a study found.
The findings build on previous research, which found that other anti-convulsant drugs are also linked to an increase in birth defects. Typically, 2-3% of babies are born with abnormalities, but among women taking epilepsy drugs, the figure is 4-8%. Birth defects were more common when women were receiving high doses of more than one drug.
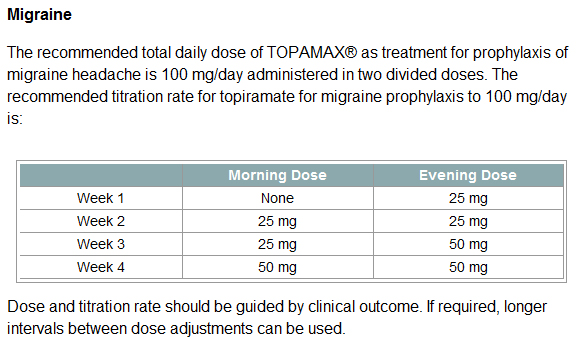
The recommended total daily dose of TOPAMAX® as treatment for prophylaxis of migraine headache is 100 mg/day administered in two divided doses.
The recommended total daily dose of TOPAMAX® as adjunctive therapy in adults with partial seizures is 200-400 mg/day in two divided doses, and 400 mg/day in two divided doses as adjunctive treatment in adults with primary generalized tonic-clonic seizures.
Qnexa contains:
* Full strength formula: 15 mg of Phentermine IR and 92 mg of Topiramate CR
* Mid strength formula: 7.5 mg Phentermine IR and 46 mg Topiramate CR
* Low strength formula: 3.75 mg Phentermine IR and 23 mg Topiramate CR
Thus, VVUS needs statistics for 46 mg of Topiramate once daily because Qnexa mid was dosed as phentermine 7.5 mg and topiramate 46 mg, po once daily.
So, twice less than for prophylaxis of migraine headache: 100 mg/day administered in two divided doses i.e. 2 pills (immediate release) 50 mg each.
Since birth defects were observed only with high doses of Topiramate (200-400 mg/day) VVUS has a chance to get very good statistics for low doses. Best results will be no any birth defects for 100 mg/day.
Is it possibly? Why not?
Statistics said that typically, 2-3% of babies are born with abnormalities, but among women taking epilepsy drugs, the figure is 4-8%.
Birth defects were more common when women were receiving high doses of more than one drug.
Topiramate to prevent migraines is 50 mg pill that taken 2 times per day. So, Cmax (maximum drug concentration in blood) should be very low after 1 pill. I'm not sure how many pregnant women were taking Topiramate because of migraine.
Category C is given to medicines that have not been studied in pregnant humans but do appear to cause harm to the fetus in animal studies. Also, medicines that have not been studied in any pregnant women or animals are automatically given a Pregnancy Category C rating.
Topamax was given a pregnancy Category C rating because of potential problems in animal studies. When given to pregnant rabbits, rats, or mice, Topamax caused birth defects, miscarriages, and decreased fetal weight. In small studies of Topamax in pregnant women, a few birth defects were seen, especially hypospadias (a birth defect in boys in which the opening to the urinary tract is not located at the tip of the penis). It is important to understand that in these studies, Topamax was taken with other seizure medications (which may have caused the birth defects).
Thus, may be these birth defects were induced not by Topiramate but other seizure medications.
Dr. Koren stated at Qnexa panel: "after the analysis I showed you, the systemic review of the existing data today do not suggest an increased risk of major malformations with topiramate in pregnant woman when compared to untreated epilepsy, for the reasons I explained to you. The pattern of reported malformation in the spontaneous system is consistent with what you expect. There was no excess of any particular malformation." Entire document is here (see pages: 73-83).
Vivus should collect the statistics showing: women that were using Topiramate (low doses for migraine) during pregnancy had
children that were born without any birth defects or these birth defects were at the conventional statistical level (no any drug at pregnancy).
Prevalence rates reported for live births for Cleft lip with or without Cleft Palate (CL +/- P) and Cleft Palate alone (CPO) varies within different ethnic groups.
The highest prevalence rates for (CL +/- P) are reported for Native Americans and Asians. Africans have the lowest prevalence rates.
* Native Americans: 3.74/1000
* Japanese: 0.82/1000 to 3.36/1000
* Chinese: 1.45/1000 to 4.04/1000
* Caucasians: 1.43/1000 to 1.86/1000
* Latin Americans: 1.04/1000
* Africans: 0.18/1000 to 1.67/1000
Rate of occurrence of CPO is similar for Caucasians, Africans, North American natives, Japanese and Chinese. The trait is dominant.
Prevalence of "cleft uvula" has varied from .02% to 18.8% with the highest numbers found among Chippewa and Navajo and the lowest generally in Africans.
I think that VVUS needs several months to collect and analyze these data.
If data is OK Qnexa medium dose will be first (from 3 current candidates) obesity pills approved by FDA.
Why not ARNA lorcaserin?
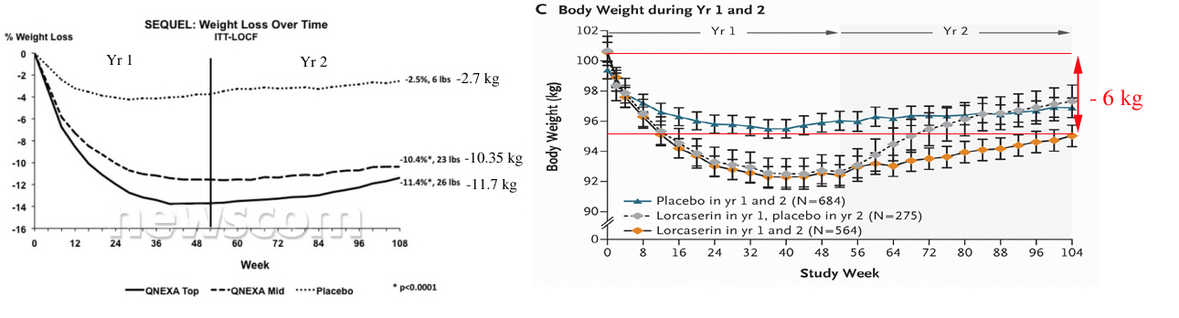
Compare 2 year efficacy results for both drugs (Qnexa, left and Lorcaserin, right).
Figures copied from
http://www.newscom.com/cgi-bin/prnh/20100921/CG67932 and published paper.
So, obviously that Qnexa is at least 2 times stronger than Lorcaserin.
What about safety?
Both drugs were rejected by FDA panel because of safety and side effects. And some panelists asked about 2 years safety for Qnexa. Now we know that "treatment-emergent serious adverse event rates in SEQUEL were low (top-dose = 4.1%; mid-dose = 2.6%) and similar to placebo (4.0%), with no drug-related serious adverse events reported."
In case of "panel of outside advisers to the FDA voted against recommending the drug for approval, citing concerns about tumors seen in rats in early-stage testing of the drug. … Of particular concern was the increase of mammary tumors in rats treated with lorcaserin".
So, Qnexa side effect such as headaches, mouth dryness, sleep disorder, tingling, diarrhea, etc. Other common side effects from Qnexa include a sensation of prickling in the skin and constipation. The combination treatment seems to have less cognitive side effects and lower discontinuation rates than topiramate alone.
Versus lorcaserin potential tumor...
ARNA aftermath:
"In October, the health regulator rejected Arena's lorcaserin, citing cancer risks. It also sought a three-month study to establish a causal relationship between the drug, mammary tumor development in rats and elevation in prolactin -- a hormone released by the pituitary gland.
On Thursday, the company said the U.S. Food and Drug Administration requested several additional studies, including a separate 12-month trial in female rats to correlate a temporary elevation in prolactin levels to mammary tumors.
Arena, however, said it can resubmit the lorcaserin NDA by the end of 2011."
No ways, may be the end of 2012 at least.
VVUS aftermath:
"The U.S. Food and Drug Administration asked Vivus to examine whether it can use existing databases to determine the risk of oral cleft in children whose mothers took topiramate to prevent migraines"
No any trials, just to analyze the medical statistics. 2-3 months usually is enough. If data is good (no oral cleft risk for Topiramate low doses), Qnexa mid dose could be approved by the end of Q3-4 2011.
OREX aftermath:
"Orexigen Therapeutics Inc. said the Food and Drug Administration is concerned about the heart side effects of its drug Contrave and will require a new study. Clinical trials to study rare events like heart attack can take years to conduct and cost millions of dollars."
So, 2-3 years delay (at least) i.e. FDA approval in 2013-2014 (if any).
Final question is: Who is possible winner in this obesity race?
Disclosure: I already opened VVUS positions (yes, again even after giant VVUS losses). It's not about "to fall in love with stock". Just the combination of science/fundamental analysis showed that VVUS Qnexa can be approved by FDA as obesity treatment pills (even if with "black box" for pregnant women it will be great). And don't forget about second VVUS drug Avanafil. Actually very effective one (Avanafil has an 80% success rate and works faster than others).
So, what possible pps for VVUS if FDA approve both Qnexa (obesity) and Avanafil (ED)? Now it's $7.78. Could be $77.80 after both approvals? Why not?
April 11, 2011 by BiotechInvest
QNEXA® Phase 3 Data in The Lancet Show Significant Weight Loss and Broad Improvements in Co-Morbidities
MOUNTAIN VIEW, Calif., April 11, 2011 /PRNewswire/ -- VIVUS, Inc. (NASDAQ:VVUS - News) today announced that detailed results from the 56-week CONQUER study were published in The Lancet evaluating the efficacy and safety of investigational drug QNEXA in 2,487 patients across 93 sites in the US. Data published in the peer-reviewed journal provided an in-depth look at weight loss and improvements in the full spectrum of co-morbidities studied as secondary endpoints, including cardiovascular, metabolic and inflammatory risk factors.
"Obesity is a serious medical condition associated with increased mortality from cardiovascular diseases, diabetes, cancer and other diseases, yet there is a lack of treatment options for the one-third of American adults who are obese," said Kishore Gadde, MD, director of obesity clinical trials at Duke University and lead investigator. "Half the patients in the study had at least three co-morbidities including diabetes, representing a population with the greatest medical need for weight loss. We observed significant weight loss, improvements in co-morbidities and a reduction in the need for concomitant medications in patients treated with QNEXA."
Specific results for all patients through 56 weeks as published in The Lancet are as follows:
Weight Loss
* Average weight loss for QNEXA patients who completed the CONQUER study on the study drug was 28 pounds and 22 pounds with top-dose QNEXA and mid-dose QNEXA, respectively, compared to 4 pounds in the placebo group;
* In the ITT-LOCF analysis, least-squares mean percent weight loss at week 56 was -7.8%* and -9.8%*, respectively, for the mid and top dose as compared to -1.2% for the placebo group;
Blood Pressure
* Reduction in systolic blood pressure of -4.7 mm Hg (p=0.0008) and -5.6 mm Hg (p<0.0001), respectively, for the mid and top dose as compared to -2.4 mm Hg for the placebo group;
* Reduction in diastolic blood pressure of -3.4 mm Hg (p=0.1281) and -3.8 mm Hg (p=0.0031), respectively, for the mid and top dose as compared to -2.7 mm Hg for the placebo group;
* More patients had a reduction in the number of blood pressure medications with QNEXA treatment compared to placebo.
Lipids
* Improvements in HDL cholesterol of 5.2% (p<0.0001) and 6.8% (p<0.0001), respectively, for the mid and top dose as compared to 1.2% for the placebo group;
* Reduction in LDL cholesterol of -3.7% (p=0.7391) and -6.9% (p=0.0069), respectively, for the mid and top dose as compared to -4.1% for the placebo group;
* Reduction in triglyceride levels of -8.6% (p<0.0001) and -10.6% (p<0.0001), respectively, for the mid and top dose as compared to an increase of 4.7% for the placebo group.
Metabolic Parameters
* Reduction in fasting insulin of -24.0 pmol/L (p=0.0004) and -27.6 pmol/L (p<0.0001), respectively, for the mid and top dose as compared to an increase of 5.1 pmol/L for the placebo group;
* Fewer non-diabetic patients on QNEXA progressed to type 2 diabetes; relative risk (vs placebo) was 0.47 (0.25 – 0.88) with top-dose QNEXA;
* More patients in the placebo group required an increase in the number of antidiabetic drugs than those treated with QNEXA.
More patients completed one year of treatment in the QNEXA groups, mid dose (69%) and top dose (64%) respectively, as compared to 57% in the placebo group. QNEXA therapy was well tolerated, with no unexpected adverse events. The most common side effects were dry mouth, paresthesia (tingling), constipation, insomnia, dizziness and dysgeusia (altered taste). Rates of serious adverse events were similar across treatment groups: 4% with placebo, 3% with mid-dose QNEXA and 5% with top-dose QNEXA. Most adverse events were seen early in treatment and there was a low dropout rate due to adverse events, 12% and 19% for mid and top dose respectively, compared to 9% for placebo.
About the CONQUER Study
The CONQUER study included 2,487 overweight and obese patients (1,737 females and 750 males) with high blood pressure, high cholesterol or type 2 diabetes across 93 centers in the United States. The average baseline BMI of the study population was 36.6 kg/m2 and baseline weight was 227 pounds. The study was a randomized, double-blind, placebo-controlled, 3-arm, prospective trial with patients randomized to receive once-a-day treatment with mid-dose QNEXA, top dose QNEXA or placebo. Patients had a 4-week dose titration period followed by 52 weeks of treatment. Throughout the 56-week treatment period, all patients were advised to follow a modest lifestyle modification program including reduction of food intake by 500 calories per day. Patients were actively managed to standard of care for weight-related co-morbidities, which included the ability for physicians to adjust or alter medications for these conditions, including in the placebo group.
Qnexa again showed perfect results for obesity. This time we have some strong evidences that this drug also decreases LDL cholesterol,
improves HDL cholesterol, decrease the triglyceride levels and reduces blood pressure. Indeed it's perfect combination. Definitely Qnexa is a winner in obesity drug race. FDA should approve it may be even with "black box" restriction for pregnant women.
Disclosure: I bought VVUS today (long-term investment).
April 28, 2011 by BiotechInvest
I sold VVUS today and will buy it back during next days. Need money to buy SPPI.
May 03, 2011 by BiotechInvest
VVUS did exactly what they must to do long time ago:
UPDATE 1-Vivus to submit Qnexa application as limited indication, shares up
Mon May 2, 2011 5:13pm EDT
* Says decided goals of Qnexa study with FDA
* Plans to resubmit Qnexa marketing approval in Q4 as lmtd indication
* Q1 loss per shr $0.12 vs est $0.15
* Shares up 6 pct after-market (Follows alerts)
May 2, 2011 Drugmaker Vivus Inc said it plans to resubmit its application to market its obesity drug in the fourth quarter as a limited indication, sending its shares up 6 percent after the bell.
The company also said it met with the Food and Drug Administration on April 14 and agreed on the goals of a feasibility study on Qnexa.
In January, the FDA asked Vivus to assess the feasibility of analyzing existing databases to determine the historical incidence of oral cleft -- commonly known as cleft lip -- in offspring of women treated with topiramate.
Vivus's Qnexa combines topiramate, which has been used for seizures and migraines, with another drug, phentermine.
The resubmission of marketing approval for Qnexa for a limited indication would include only men and women of non-child bearing potential.
Vivus also narrowed its quarterly loss and beat market estimates, as it reduced research and development spending on its drugs.
Vivus reported a first-quarter net loss of $9.9 million, or 12 cents per share, compared with a net loss of $18.8 million, or 23 cents per share, a year earlier.
U.S. Obesity Trends
Trends by State 1985–2009
Obesity is defined as a body mass index (BMI) of 30 or greater. BMI is calculated from a person's weight and height and provides a reasonable indicator of body fatness and weight categories that may lead to health problems. Obesity is a major risk factor for cardiovascular disease, certain types of cancer, and type 2 diabetes.
During the past 20 years there has been a dramatic increase in obesity in the United States. In 2009, only Colorado and the District of Columbia had a prevalence of obesity less than 20%.
"Almost two-thirds (about 66%) of U.S. adults age 20 or older are overweight -- about 62% of women and around 71% of men. Nearly one-third (about 31%) of American adults are so overweight that they are considered obese, meaning they have a BMI greater than 30; that breaks down to about 33% of women and 30% of men."
Why these numbers are important?
Recent VVUS bashers used next arguments: "And more women than men are believed to be willing to pop a diet pill. Yoo notes that in Qnexa Phase III trials, 70 percent to 83 percent were women, "which is typical for obesity therapy."
It's absolutely incorrect statistics, in U.S. we have more overweight men (71%) than women (62%). Women just want to be slim much stronger than men, so they go to any clinical trial to fight an obesity. Sure, that obesity men will take prescription pills to fight with obesity and future problems that this disease promotes (diabetes, high blood pressure, strokes and etcetera).
And don't forget about VVUS pipeline:
2 drugs were successful in phase III trials and both drugs are potential blockbusters with future billion sales...
At that Market Cap: 685.40M and pps $8.36?
Big pharmas must be already in line to offer VVUS buyout for 100% premium. If they not do it.... It will be very stupid.
"Pfizer will lose its patent for Viagra on March 27, 2012, according to the U.S. Patent and Trademark Office, at which point any drug company will be able to make and sell a cheap "generic" version of the blockbuster erectile dysfunction (ED) drug. Doctors and lawyers believe that the expiration of Pfizer's monopoly on the drug will be good news for patients, as it will force competition between Pfizer's Viagra and the new generic versions, dramatically driving down the price not only of Viagra but also of Eli Lilly's Cialis and Bayer's Levitra."
Avanafil has going for it is the time it takes for the drug to start working and its reported low side effects. Times seem to vary depending on what reports you read but Cialis is reported to work in as little as 30 minutes but most take it somewhere between 30minutes and 12 hours before because it takes over 30 minutes to reach full plasma levels. One is to take Viagra approximately 1 hour before and Levitra about one hour before. All have their benefits and negatives.
But Avanafil is reported to start working in 30 minutes or less (a large percentage of the men who participated in the trial report it working in as little as 15 minutes and feeling the effects for as long as 6 hours). It claims to be quicker than 'the others'.
This may be the key to Avanafil in a market already having effective ED medications. Avanafil also reportedly stays in the blood less time which could potentially lead to fewer side effects than other treatments. Some of the side effects in phase 3 trials where a low but consisted of headache, skin flushing, nasal congestion but no visual imparities like that seen in sildenafil (Viagra)
* FDA filing for Avanafil looks to be in late 2010 or early 2011
* Market Launch expected in early 2012.
Conclusion: it seems like that VVUS won this obesity race, I bought shares back for long-term investment this time.
02.21.12 by BiotechInvest
Today the funniest story happened with VVUS stock: pps drop 12% because "graduate student pursuing a PhD in pharmaceutical sciences and pharmacogenomics. By night, he's the "FDA Panel Whisperer" -- the creator of FDATracker.com, a unique web site that predicts outcomes of FDA advisory committees based on the historical voting records and behavioral analysis of participating panelists."
"Lee has crunched the data on the 22 panel members who will sit in judgment of Vivus(VVUS_) and its obesity drug Qnexa tomorrow and he's not encouraged. His prediction: 10 experts will vote to recommend Qnexa approval but the remaining 12 will vote against. That's a very close vote, made more so by Lee's acknowledgement that one or two votes could swing in either direction, according to his analysis."
But funnier or more funny that :analyst" AF from TheStreet bought these cheap speculations and this "predictions" crashed VVUS stock today.
Crazy world, crazy people...
The truth is that some funds used this designed rumor and bought 11M shares 12% cheaper today.
And also truth is that VVUS has a effective and safe obesity drug and both FDA and USA government want it now to fight the epidemy of obesity in USA.
This idiotical prediction said:
"FDA panel members predicted to vote against Qnexa's approval, according to Lee:
Erica Brittain, Ken Burman, David Capuzzi, Robert Clancy, Janet Cragan, Katherine Flegal, Ed Gregg, Mike Lauer, Elaine Morrato, Sonja Rasmussen, Lamont Weide, Almut Winterstein."
Of course these guys read it today and now they are under giant pressure: if you vote "no" you are so predictable individual that even "graduate student pursuing a PhD in pharmaceutical sciences" can calculate your vote; if you vote "yes" - seems like you are afraid that people will think that you vote in defiance of "published prediction"
What should do these panel members? Abstain from voting? They are in logistical trap now and any vote will be explained badly for them.
They should not say the names of possible negative and positive voters:
FDA panel members predicted to vote for Qnexa's approval, according to Lee:
Melanie Coffin, Eric Felner, Allison Goldfine, Jessica Henderson, Sanjay Kaul, Michael Rogawski, Robert Smith, Myrlene Staten, Abraham Thomas, Susan Yanovski.
FDA panel members predicted to vote against Qnexa's approval, according to Lee:
Erica Brittain, Ken Burman, David Capuzzi, Robert Clancy, Janet Cragan, Katherine Flegal, Ed Gregg, Mike Lauer, Elaine Morrato, Sonja Rasmussen, Lamont Weide, Almut Winterstein.
Conclusions: VVUS should sue both FDA Panel Whisperer and TheStreet for attempt to manipulate FDA panel vote. Because this is an open vote panel and all panel members are under the "logistical trap" pressure now.
02.22.12 by BiotechInvest
Well, everybody knows now that FDA will approve Qnexa (because USA government wants it). In April 17, 2012 USA will have an effective obesity drug. Then in 04/29/2012 VVUS has an erectile dysfunction Avanafil PDUFA (>80% probability of approval). Already now VVUS cap is $2B (pps $21) and with Avanafil approval it will doubled i.e. $4B and pps $40-50.
So, I'll keep VVUS before April.
GL and ABG (always be green)
03.08.12 by BiotechInvest
VVUS is under attack of Jefferies's ass-kissers
Just read this stupid and ugly lie:
"On February 27th, the FDA advisory committee by a vote of 20-2, recommended the approval of Qnexa for the treatment of obesity, including weight loss and weight maintenance for obese patients.
The final decision from the FDA to approve the drug or not is April 17th, 2012.
What I am about to say is sure to draw a lot of angst; I am one that believes the drug will not be approved this time around. It is not too often the FDA rejects a recommendation from its own advisory panel, but I think this is a rare time that it not only should, but will.
First off, there has been no safety data I have seen on the drug's effect on children, so we can safely assume the drug will not be approved for pre-pubescents. In 2010, the advisory committee rejected Qnexa over concerns of increased heart rate and birth defects. That panel concluded that the drug's weight-loss benefits did not outweigh its potential risks.
In clinical trials, Qnexa led to 10% weight loss over a year, but it was also linked with increased heart rate and birth defects when taken during pregnancy. Is a 10% weight loss worth the risk of increased heart rates and birth defects? I believe the FDA is on a slippery slope with Qnexa. This is not an important orphan drug designed to literally save lives, where the adverse risks would be acceptable. Furthermore, if Qnexa showed a weight loss over 15%, then we could weigh the benefit verses risk with more weight.
My opinion on VVUS: Short sell it."
"no safety data I have seen on the drug's effect on children" - idiot, this is not drug for children.
"Qnexa led to 10% weight loss over a year, but it was also linked with increased heart rate and birth defects when taken during pregnancy." - complete idiot, Qnexa never was linked to birth defects when taken during pregnancy. Just read FDA papers before say such stupid statement. It said:
"There were 34 pregnancies in the PHEN/TPM clinical development program with an average gestational age at diagnosis of 5.4 weeks. Of the 19 pregnancies carried to term, newborn examinations did not reveal any major malformations."
Only Qnexa's component topiramate was linked to the risk of oral cleft in children whose mothers took it to prevent migraines.
But Qnexa contains topiramate in CR (controlled-release )form and Cmax is much lower than for migraine Topiramate IR (Immediate-Release). I hope FDA knows what is PK/PD profiles of CR and IR drugs.
Jefferies bumped its price target on Vivus from $11 to $12 following Q4 results but kept its Underperform rating.
Well, some funds paid them for VVUS pps bashing. And they are paying for any stupid lie about VVUS.